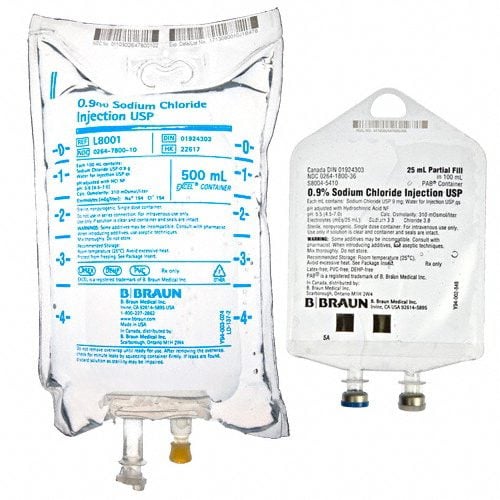
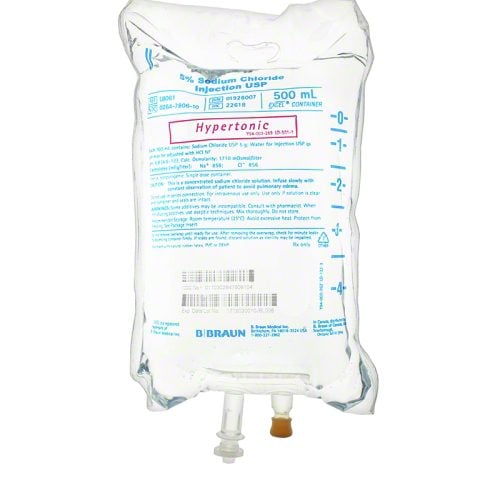
Sodium Chloride Injections are a safe and effective way to replenish electrolytes in the body, aiding in proper hydration. This effective treatment helps reduce swelling and provide relief from fatigue, weakness, and cramping. Sodium Chloride Injections are a reliable and efficient method for administering electrolytes in a convenient intravenous format.
Features
- Sodium Chloride Injections USP are sterile, nonpyrogenic, isotonic and contain no bacteriostatic or antimicrobial agents.
- These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes and water for hydration.
Options available
BBNL8001 – Sodium Chloride Injections | 0.9% | 500ml [24/case]
BBNL8002 – Sodium Chloride Injections | 0.9% | 250ml [24/case]
BBNL8021 -Sodium Chloride Injections | 0.45% | 500ml [24/case]
BBNL8061 – Sodium Chloride Injections | 5% | 500ml [24/case]
BBNS8004-5264 – Sodium Chloride Injections Partial Additive Bag | 0.9% | 100/150ml [64/case]
BBNS8004-5384 – Sodium Chloride Injections Partial Additive Bag | 0.9% | 50/100ml [84/case]