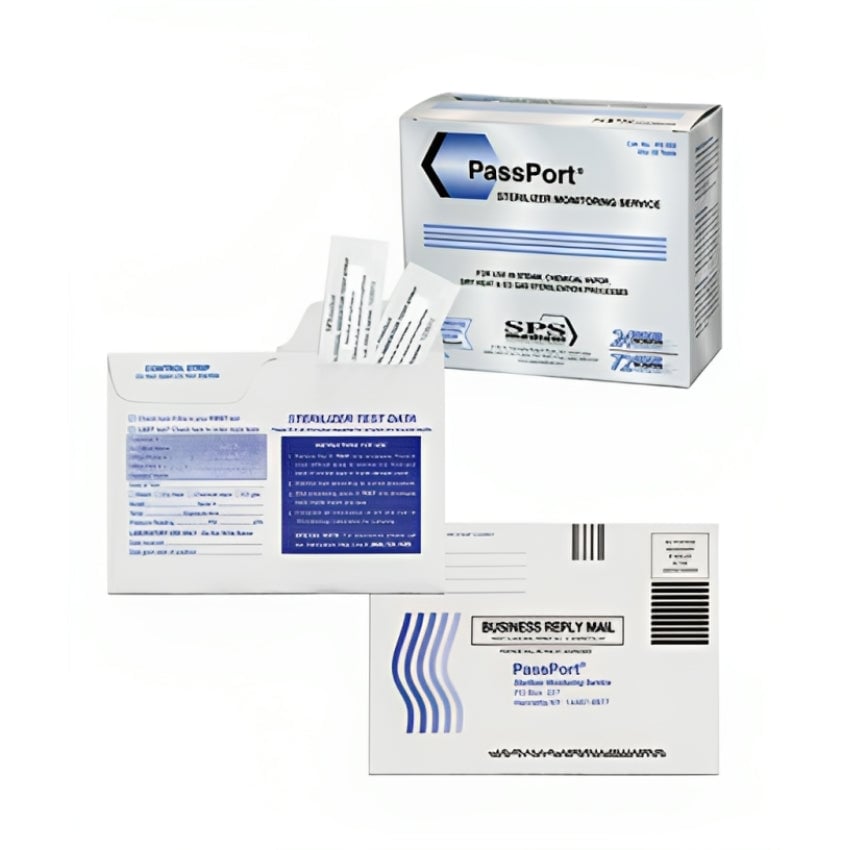
Crosstex PassPort™ Sterilizer Monitoring Service offers a comprehensive solution for healthcare facilities to ensure effective sterilization. It includes testing materials, shipping, and a secure online portal for tracking and reporting results. This service ensures compliance with industry standards and regulations, providing a critical component of infection control protocols and a sense of assurance for patients and healthcare professionals.
Features
- Two test strips and one control strip are provided for users to spore test multiple locations within the sterilizer chamber.
- Dry Heat test results are documented and reported back to the customer after 7 days incubation.
- Steam test results are documented and reported back to the customer after 24 hours incubation.
- Chemical Vapor test results are documented and reported back to the customer after 72 hours incubation.
Sold by
PassPort™ Sterilizer Monitoring Service|12/box